
The modernization of a universities General Chemistry laboratory program can be a daunting and yet necessary task. This is especially true when you have 2000+ students taking General Chemistry every year. The University of Tennessee, Knoxville decided it was time to modernize their General Chemistry Lab Program for them to remain competitive. They knew that Electronic Data Collection Technology needed part of this process, and needed to be integrated into the labs. I am happy to say The University of Tennessee, Knoxville decided MeasureNet's Electronic Data Collection System was the technology selected in the redesign of their General Chemistry Laboratory Program.
“The laboratory makeover not only benefits the first-year students, but will help improve their preparation and success as they go on to take upper-level chemistry courses, “
Al Hazari, Director of Undergraduate Laboratories.
The folks here at MeasureNet have been hard at work integrating new probes into our system and creating new experiments. In this blog entry we'll be introducing the following new experiments and probes:
- Thermometric Titrations
- Conductivity Probe & Experiment Options
- Colorimetric Titration Hardware
Thermometric Titrations
The newest software integrates the drop counter and temperature probe and now has the capability to conduct experiments with Temperature vs Drops. This allows users to conduct Thermometric Titrations.

Conductivity Probe & Experiment Options
MeasureNet now offers a 4 range high resolution conductivity probe. MeasureNet provides users the ability to conduct experiments with Conductivity vs Time and use the drop counter for Conductivity vs Volume for Conductometric Titrations.

Colorimetric Titration Hardware
MeasureNet systems now have the ability to conduct colorimetric, Fluorometric, turbidometric and chemiluminescent titration experiments. When ordering colorimeters, customers can specify if they would like them customized for photometric titrations. The kit includes a colorimeter, a base stand, a pump with power supply, and custom-made flow cell that pair with the MeasureNet colorimeter and drop counter.
The folks here at MeasureNet have been hard at work integrating new probes into our system and creating new experiments. In this blog entry we'll be introducing the following new experiments and probes:
- Dual Probe Experiments (Pressure, Temperature & Voltgage)
- Thermocouple Temperature Probe
- Melting point Experiment
Dual Probe Experiments
MeasureNet now has the ability to collect data from 2 of the same probe if using the voltage, pressure, or temperature probes. New experiment options have been added to each probe's menu to allow dual probe collection. In order to use the dual probe option, customers will need the MeasureNet dual probe adaptor (pictured below).

Thermocouple Temperature Probe
MeasureNet also offers a thermocouple type J temperature probe now for applications that fall outside the range of our standard temperature probe. The new thermocouple probe has a temperature range from -180C to +475C.

Mel-Temp Switch & Experiment
Along with the thermocouple probe, MeasureNet now offers a solution that will make Melt Temp experiments easier. During a Mel-Temp experiment, you can use the Melt Temp Switch to keep track of a small range of temperatures when your compound nears melting temperature. You can then use print code 400 to print an overall temperature graph along with a graph that focuses on the range where the sample started to melt.

Make sure to keep a lookout for the next set of probes and experiments. We still have more to show.

MeasureNet is soon to release the newest versions of our software lineup, and we've decided to give you an in-depth tour into our latest features and upgrades in a five part series.
Look out for our blog posts as we give you a rundown of all our new features.
- Part 1 - A Better User Experience
- Part 2 - Dual Spectroscopy
- Part 3 - New Chemistry Experiments & Probes 1
- Part 4 - New Chemistry Experiments & Probes 2
- Part 5 - Real-Time Data to the Cloud & Remote Monitor Experiments
Part 1 outlines all the new user inter interface elements that make the MeasureNet software even easier to use than before.
Part 2 details the options with our new Dual Spectroscopy features, which now allows you to have two networked spectrometers for each of your network.
Parts 3 & 4 cover all of our new probes and experiments available:
- Melting Point Temperature Experiment
- Dual Probe Experiments for Pressure, Voltage, and Temperature.
- Thermometric Titration Experiment
- Type J Thermocouple Probe
- Conductivity Probe
Part 5 runs through all of the elements added to the MeasureNet Lab Software and LabKonnect Data Storage site that are now part of our new Cloud Based Real-Time Extended Monitoring and Experiments feature, which allows you to remotely monitor long term experiments from any browser.
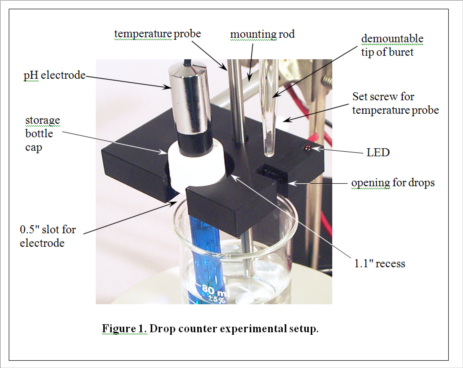
Development of MeasureNet’s optical drop counter began in 2000. Our goal was not only to automate, simplify and shorten the time for pH titration experiments, but also to improve the accuracy of students’ measurements. During its development, various innovative features were added to the design, as seen in Figure 1. MeasureNet’s Multi-Functional Drop Counter holds both a pH probe and a temperature probe, reducing the hardware needed to set up pH titration experiments, and the geometry was optimized to allow the use of small beakers and samples.
Before the days of optical drop counters, simple wire electrical conductivity devices were the only automated and economical solutions for pH titrations in the teaching laboratory. A drop from the buret would make contact with two bare wires positioned closely together, creating a conductive current path. An electronic circuit would then convert this current into a pulse that was counted. A student could then calculate volume based on the number of drops and the average drop size, determined in a separate experiment. The MeasureNet system automatically determines the average drop size in each titration.
The most common method of performing pH titrations is the manual method. The drawbacks to this method:
Time consuming, giving students time to perform few titrations in a lab period.
-
Larger reagent volumes required, making titrations more expensive in terms of reagent usage and disposal.
-
Requires repetition for students to master endpoint detection with reasonable precision.
The 22nd BCCE at Penn State University marks the 10th anniversary of MeasureNet Technology’s introduction of the Optical Drop Counter to the chemical education community. During this decade, the MeasureNet drop counter has become a star in chemistry labs around the country and the world, performing a variety of functions even its designers had never imagined. MeasureNet’s drop counter allows students to leap past the typical pH titration experiment and engage in other interesting and educational titration methods like thermometric, potentiometric, amperometric and colorimetric titrations.
MeasureNet Technology's introduction of the industry’s first Multi-Functional Optical Drop Counter technology in 2002 at the 17th BCCE at Western Washington University obviously caught the attention of the conference attendees and venders. The MeasureNet drop counter has emerged as the industry standard, copied by all its competitors, such as Vernier Software & Technology, Pasco Scientific and MicroLab Inc. MeasureNet is both flattered by this attention and inspired to do more to bring innovative technology into the teaching laboratory.

WVXU Podcast link by Ann Thompson: Focus on Technology: Bugs Cleaning Wastewater
Cincinnati scientists are engineering special bugs that will clean wastewater and create energy. Ann Thompson takes you into the lab where this is happening in Focus on Technology.
By Ann Thompson
MeasureNet Research Applications
MeasureNet is renowned for putting cutting-edge technology into the hands of students but MeasureNet may someday be famous for helping scientists find new and innovative ways for dealing with the world’s most difficult problems.
The technology provided by MeasureNet combines a high-resolution measurement workstation with their LabKonnect cloud-based software to create a system that allows researchers to monitor their experiments from anywhere. Researchers access information from the cloud, important in experiments lasting for a week or longer.
LabKonnect will also alert the researcher via text message if something has gone wrong. The researcher specifies a range; the system notifies team members if data goes beyond that range. Scientists no longer spend valuable time and resources babysitting experiments.
This technology was put through its paces recently, when MeasureNet teamed with Dr. Dan Hassett, who creates special bugs that will clean wastewater and create energy. Hassett, molecular genetics professor at the University of Cincinnati, thinks he has found a way to convert sewage into clean water and energy.
Wastewater has stored up energy in the form of pollutants. Hassett is developing bacterial robots, or “bactobots,” that break down these pollutants and release the energy. Sewage treatment plants become biological fuel cells that produce both clean water and energy.
The bactobots are tiny, only about three microns long, but they generate about 400 milivolts with fluctuations as high as 700 milivolts as they clean the water. Hassett increases the amount of power a bactobot can generate through a series of genetic mutations. Measuring the output of these miniscule bacteria is a big job, and that’s where MeasureNet steps in.
MeasureNet helped Dr. Hassett monitor the voltage and current output of biological fuel cells. Typically, the ouput voltage fluctuates and over the course of four days. Thanks to the sensitive measuring equipment and cloud capabilities provided by MeasureNet technology, Hassett found the existence of a second particular bacterium actually increased output over those four days.
While the output from a single bactobot is small, the impact of these biological fuel cells could break one of humanity’s most vexing vicious cycles. Wastewater treatment plants are the single largest consumer of energy, and the second largest user of water is energy production. Introducing a bacteria that would simultaneously clean water and produce energy would be monumental.
MeasureNet is at the forefront of hands-on laboratory technology, both in the classroom and in the research lab. Like technology itself, MeasureNet continuously develops new ways to enhance the lives and learning of students, scientists and everyday people.

Carl Sagan once said, “It’s suicidal to create a society that depends on science and technology in which no one knows anything about science and technology.”
The United States is slipping behind other countries in awarding postsecondary STEM degrees. Between 1998 and 2006, the total number of STEM degrees grew by 23 percent in the United States. During this same period, Poland increased its number of STEM degrees by 144 percent and Taiwan boasted a 178 percent increase. China’s number of postsecondary STEM degrees exploded by a stunning 200 percent. In 2006, China awarded nearly double the number of postsecondary STEM degrees gained in the United States. The USA needs more STEM degrees if it hopes to compete in tomorrow’s global economy.
More STEM degrees mean more STEM jobs. Increasing the number of jobs in STEM fields is critical to the economic prosperity of both individuals and communities. Generally speaking, STEM jobs are some of the highest paying positions, with wages significantly above the US average. STEM jobs represent one of the fastest-growing segments of the job market, both in the United States and globally. Between 2008 and 2018, the number of STEM jobs is expected to have grown by 17 percent.
STEM occupations are associated with lower unemployment rates when compared to other professions. Students who graduate with a STEM degree but pursue jobs in non-STEM fields also make more money than those with degrees in other fields. Cities across the nation try to attract STEM professionals because civic leaders understand that the high pay and economic stability associated with STEM jobs benefits the economic and social fabric of the community.
The main pathway to a lucrative career in STEM fields is through postsecondary education. Getting bright minds into postsecondary programs poses a two-fold challenge to secondary educators – sparking interest in pursuing further education in STEM studies and properly preparing students to engage in higher learning. In most K–12 systems today, math and science subjects seem to have little to do with the real world. Students often ask, “When will I ever use this knowledge?” Students just don’t seem interested in STEM and educational institutions fail those few students leaning towards a STEM degree.
The National Governors Association explored this challenge in the December 2011 updated version of Building a Science, Technology, Engineering and Math Education Agenda. This committee examined the goals of the STEM agenda and outlined why this agenda is so important to the states and to the nation. They identified weak links in the system and outlined strategies to implement state-wide STEM agendas in ways that excite students about STEM studies and careers while giving them the tools to succeed in gaining a degree and securing a job.
The STEM agenda has two basic goals: expand the number of students entering postsecondary STEM studies and increase STEM proficiency in the general student population. The first goal improves the technical capabilities of the nation’s workforce while the second goal helps students implement concepts and problem-solving strategies gained through STEM studies into their everyday lives.
Proficiency in STEM facts, principles and techniques are just as beneficial to future employees of big-box stores as they are to the physicians and engineers of tomorrow. Integrating STEM concepts and hands-on learning into the regular curriculum will help the general student population hone critical thinking skills to recognize, evaluate and solve problems not related to science, technology, engineering or mathematics.
This study identified five areas that states are trying to improve upon in an effort to increase the number of interested students qualified to pursue postsecondary STEM studies. Inconsistent state standards, a shortfall of qualified instructors and failure to motivate student interest all prevent students from pursuing postsecondary studies in STEM. This study also suggests that students are not prepared for postsecondary STEM study because they lack hands-on learning and experience with laboratory equipment. Worse yet, many current postsecondary STEM studies do not properly prepare students to work in STEM fields.
Fortunately, the report by the National Governors Association contains solid research that provides direction to increasing the presence of STEM tools in your learning laboratory in a way that propels your students into postsecondary studies and high paying STEM jobs. Building a Science, Technology, Engineering and Math Education Agenda says that several studies correlate strong preparation in high school with improved STEM degree completion rates. Furthermore, certain high school instructional practices seem to be more effective than others, including doing hands-on experiments in science. Encouraging high school students to form workgroups also improves postsecondary STEM outcomes.
Use informal learning to help students make the connection between STEM classes and real-world applications. Field trips to museums, science centers and other public and private institutions showcase job opportunities for those with degrees in STEM fields. Many of these institutions provide hands-on activities using the same equipment professionals employ. The connections between STEM classes and real-world applications are reinforced when your students can then use these same pieces of equipment in their own classroom laboratories.
The National Governors Association notes that a “student’s ability to enter and complete a STEM postsecondary degree or credential is often jeopardized because the pupil did not take sufficiently challenging courses in high school or spend enough time practicing STEM skills in hands-on activities.” Hands-on learning chemistry labs improve a student’s experience in secondary school, giving her confidence to succeed in postsecondary STEM studies or in an entry-level non-STEM career.
Our nation’s security and economic stability rest in the capable hands of exceptional educators just like you. It is up to STEM educators to expand the knowledge of science and technology not only in collegiate hopefuls but in the student population as a whole. Improve your students’ chances of success competing in a global economy by training them the same chemistry laboratory equipment that universities and professionals use.

The MCAN® Concept
The MeasureNet Multifunctional Chemical Analysis Network (MCAN® ) is an innovative analytical instrumentation eliminating a multitude of the obstacles that are associated with the PC-based lab systems. With the consolidation of student data acquisition workstations into a solitary network for each group of students, expensive hardware upgrades, computer viruses and the footprint of large equipment is eliminated.
The student workstations we provide interface with numerous kinds of probes as well as other apparatus that are utilized in chemistry laboratories. This allows for a broad range of general chemistry, physical chemistry and biochemistry lab experiments.
MeasureNet can be useful in a freshman chemistry lab or in advanced chemistry labs. Whether used in the freshman chemistry lab, biochemistry, STEM, or environmental chemistry laboratories, MeasureNet is an essential tool.
With MeasureNet, the student takes the measurements at their workstation and the results of those measurements can be stored and then monitored on a single central computer. This allows the instructor to follow student progress and data files. There is no need to contend with the multiple headaches that are associated with managing student lab data because MeasureNet can do it all for you.
Solutions in the Chemistry Lab with MeasureNet
- Less bench space due to energy efficient workstations with smaller foot prints.
- Securely Protected online data storage for students.
- Viruses and computer re-imaging are things of the past with MeasureNet.
- The collection of data electronically allows for more time being spent on the actual experiments.
- Eliminates the need to upgrade large numbers of computers every few years.
- The best quality, research grade chemistry probware is utilized to collect high-resolution data.
General Chemistry Lab Experiments Performed with MeasureNet
MeasureNet was designed to offer instructors considerable academic flexibility with its ability to support a wide range of experiments.
MeasureNet has the ability to support experiments for individual or collaborative student projects in a freshman chemistry lab or in an advanced lab.
The adoption of electronic data acquisition does not require the disposal of experiments that have been proven to build the students’ skills or enhance their understanding of imperative concepts. The longstanding experiments can very easily be integrated into curricula combined with the experiments appropriate for MeasureNet. Other experiments requiring conversion can be modified with the assistance of our curriculum specialists.
Title examples for Guided inquiry, Self-Directed, POGIL, Verification-style and STEM experiments utilizing MeasureNet are as follows:
- Specific Heat of a Metal
- Hot & Cold Packs- Dystan Medical Supply Company
- A Colligative Property of Solutions-Freezing Point
- Quality control at the GlassEX Company-Self-Directed
- Buffer & pH Solutions
- Proteins & Amino Acids
- Chemical Kinetics
- Gas Laws
- Voltaic Cells
- Substances Specific Heat
- Analysis of Metals Emissions
- Heat of Vaporization & Vapor Pressure
- Determining Chromium (VI) Concentrations using Absorption Spectroscopy
- Colligative Properties
- Determining the Cause of a Fish Kill Located in the Clark Fork of the Columbia River
- Identification of a Weak Unknown Acid
- Analysis of the Phosphorus in Cola
- Identification of an Unknown Metal-Self Directed
- Determining the Ka Value of a Weak Acid
- Determining the concentration of Acetic Acid in Vinegar
- Determining the Molecular Weight, with the use of the Ideal Gas Law, of a Volatile liquid
- Hess’ Law-Enthalpy of Reaction
- Analysis of the Phosphorus in Fertilizer
- Determining the Heat of Neutralization for Various Strong Bases & Acid
- Reaction Stoichiometry & Moles
- Determining a Reaction Equilibrium Constant with the Use of Absorption Spectroscopy
- Analysis of Emission of Aequeous Solutions from Group IA & IIA Metal Salts
#3) TURBIDIMETRIC / NEPHELOMETRIC LIGHT-SCATTERING SPECTROMETRY
The “Colorimeter” can also be used in the “Turbidometer” mode… or more precisely, as a “Nephelometer”, since nephelometry uses a Near-IR LED (@ 880nm) to measure the SCATTERED light at a 90° angle from the light source, as it is done on the MeasureNet unit. Tests can be made on sample solutions that have existing particulate suspensions… or a reaction can be made to produce a range of particulates for relative and direct measurements. Originally used for Clinical Chemistry applications, Turbidometry/Nephelometry can be applied to a wide range of curriculum subjects… from Organic Synthesis & Analytical Chemistry to Environmental & Biochemistry Labs. Some of these unique and educational labs are shown here:
Turbidity (“Nephelometry”) for Kinetic & Relative Comparison at Single values:
• Environmental Testing of “Settleable Solids” for River / Stream & Estuarial Waters
• Reducing Sugars by Benedict’s Test... great for Diabetics, Atkins-Diets and all the rest!
• Collect some of the DUST from your “Air”… is it okay to breathe or is something there?
• Rate of Reaction of Silver Ion + Chloride Ion to form AgCl precipitate… Kool Kinetics!
• The classic Princeton “Nassau Clock” Reaction… makes for a great teaching Lab attraction!
• Measurement of Yeast Growth in Beer… is it fermenting slow or in high-gear?
A typical colorimeter is usually a simple, single-beam optical system to measure “color” in the VISIBLE Spectrum of light and provide ABSORBANCE data at a single wavelength. These systems can cost $1,000 or more for stand-alone colorimeters that merely provide simple ABS data… unless you consider the MeasureNet Model MDBC-138 true Double-Beam, Multi-Functional “Colorimeter” based on a unique set of LEDs (Light-Emitting Diodes)... for under $500! The MeasureNet “Colorimeter” is actually FOUR (4) instruments in ONE (1) compact and rugged box… and works as a colorimeter (to make Beer’s Law Curves for VISIBLE Wavelengths), a UV fluorometer (to demonstrate fluorescence & quenching in certain organic molecules), a turbidometer (to measure the turbidity of particulate & colloidal suspensions) and a phosphorimeter (to analyze the phosphorescence [“glow”] of specific materials).
#1) BEER’S LAW ABSORBANCE & KINETIC SPECTROSCOPY
This unit can be used as a “simple” Colorimeter to demonstrate Beer’s Law using three (3) high-output VISIBLE Light LEDs at 472nm (BLUE), 525nm (GREEN) and 630nm (RED); which cover over 75% of the classic General Chemistry and Analytical Chemistry Laboratory experiments that teach spectrophotometric measurements. The wavelength coverage of these LEDs allows highly accurate relative measurement of almost all the ROYGBIV Colors. The disposable & unbreakable 10mm pathlength, near-UV plastic cuvettes included with the “Colorimeter” can be used from ~300nm (in the near-UV) to over 1000nm (in the near-IR) for analytical measurements… and come with sealing caps to preserve prepared solutions for future tests. Some of the popular Laboratory experiments for your Academic Lab curriculum programs Gen Chem, Analytical or Student Research are:
Red / Blue / Green Colorimetric ABS Data for Beer’s Law plots:
• Test for Mineral IRON in your food… check your Cereals, Breads and more
• Unsaturated FATS can be easily seen… just get a purple Color using some IODINE!
• Evaluation of Nutritional Food Proteins… React it to get a “BLUE” and see what can be seen!
• Consistency of M&M and Skittles Candy Colors: Is the "blue" true blue or “faux” to you!
• Changing “Colors” of some pH Dyes… [H+] makes them look *new* to our eyes!
• General Colorimetric Assay… for ANION (X, PO4, SO4, NO3, NO2, etc)
The MDBC-138 is a TRUE Double-Beam Optical System… and provides a reference cell to “blank” out the reagents used to create a very stable ABS reading for several Organic Reactions. Data from these Labs can be used to calculate rate constants, equilibrium factors and reaction conditions (for thermal, ionic and electrochemical variables). This is a MUST for making accurate kinetic experiment tests. A few of them from our “Library” include:
Dual-Beam ABS Data for Kinetic Measurements:
• The Iodine Test for Starches… just Hydrolyze with Amylaze to get a Kinetic Rate
• Perform REDOX “Clock” Reactions
• pH-based Hydrolysis of p-nitrophenylacetate Ester