
"Benzene"
September 20, 2006
Friedrich August Kekulé (1829-1896). German chemist. Initially a student of architecture, Kekulé studied chemistry under Liebig at Giessen and under Dumas in Paris. After brief periods in Switzerland and England (1854), and at the University of Heidelberg (1856), he became Professor of Chemistry at the universities of Ghent (1859) and Bonn (1867-1896). Acknowledged as one of the founders of classical molecular structure theory, Kekulé is best known for his proposal (along with the Scottish chemist, Archibald Scott Couper) that tetravalent carbon can undergo self-linkage to form homocatenated chains and rings (1858), and for his hexagonal cyclic structure for the benzene molecule (1865).
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati

Linus Pauling (1901-1994). American chemist. Professor at the California Institute of Technology (1927-1962), and Nobel Prize winner for both chemistry (1954) and peace (1962). Though Pauling began his career as a crystallographer, he is best known for his work on the electronic theory of the chemical bond, as summarized in his 1939 monograph, The Nature of the Chemical Bond, and for his later work on the molecular and electronic structures of large biomolecules. His name is most often associated with his scale of ionic radii (1927), his rules for rationalizing the most stable structures for complex ionic crystals (1929), his extensive use of the both the hybridization (1931) and resonance concepts (1932) in chemical bonding, and his thermochemical electronegativity scale (1932). He also gained considerable notoriety for his political activism in support of nuclear disarmament, and for his controversial advocacy of the supposed medical benefits of megadoses of vitamin C.
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati

"In the Beginning, There Was a Dog Kidney"
Friedrich Wöhler (1800-1882), German chemist. A student of Leopold Gmelin at Heidelberg and of the great Berzelius at Stockholm, Wöhler held teaching positions at Berlin and Cassel before accepting the chair in chemistry at Göttingen in 1836, where he remained for the rest of his life. An eclectic chemist, he is best known in the field of organic chemistry for his collaborative work with Liebig on the isomerism of the fulminate and cyanate radicals (1823) and on the chemistry of the benzoyl radical (1832), and for his synthesis of urea from ammonium cyanate, (1828) which was later interpreted as a decisive blow against the doctrine of vitalism. In the field of inorganic chemistry, he is best known for his isolation of the elements aluminum (1827) and beryllium (1828) and, in the field of chemical education, for the many 19th-century American chemists who came to study in his laboratory.
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati

"Annalen"
Justus von Liebig (1803-1873) Professor at Giessen (1824-1852) and Munich (1852-1873), Liebig's teaching laboratory at Giessen served as the model for the advanced training of chemists in the first half of the 19th century, including many foreign students from Great Britain and the United States, and, through his editorship (starting in 1832) of the journal, Annalen der Chemie und der Pharmacie (which is still published under the name of Liebig's Annalen), he exercised enormous influence on the early development of organic chemistry, agricultural chemistry, and physiology. He perfected organic combustion analysis (1837) and the counter-current laboratory condenser (1843), and, in collaboration with his close friend, Friedrich Wöhler, did pioneering work on the isomerism of fulminates (1823-1826) and the reactivity of the benzoyl radical (1832).
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati

Stanislao Cannizzaro (1826-1910) Professor at Alessandria (1851-1854), Genoa (1855-1859), Palermo (1860-1869) and Rome (1870-1910), Cannizzaro is best known for his discovery of the Cannizzaro reaction in organic chemistry for the conversion of aromatic aldehydes into the corresponding acids and alcohols, and his successful resolution of the atomic weight problem. Though Dalton had introduced the idea of extracting atomic weights from combining weights in 1803, he was unable to do this in a completely unambiguous fashion and, as a result, 50 years of chaos followed during which chemists used a variety of competing atomic and equivalent weight values. In 1858 Cannizzaro published a small pamphlet in which he reasserted Avogadro's earlier hypothesis (1811) that gas densities at equal pressures were directly proportional to molecular weights. Whereas Avogadro had attempted to extract atomic weights from the resulting molecular weights by using the stoichiometries of gas reactions - a procedure that could be applied only to a few elements - Cannizzaro showed how this same information could be extracted by using the gravimetric composition of an element's volatile compounds - a procedure that was virtually universal. With Cannizzaro's advance, chemists finally acquired a standard set of atomic weights and were able to determine unambiguous and universally accepted compositional formulas for their compounds.
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati
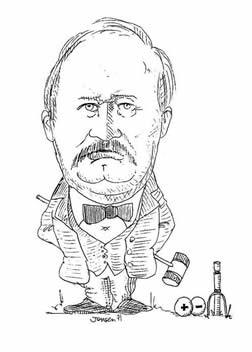
Svante Arrhenius (1859-1927). Swedish Chemist. Lecturer and Professor at the Technical Institute in Stockholm (1891-1904). Nobel Prize Winner (1903) and later Director of the Nobel Institute (1905-1927). Arrhenius is best known for his theory of ionic dissociation, which evolved out of his doctoral thesis of 1884, his ionic acid-based definitions (1887), and his introduction of the concept of activation energy in chemical kinetics (1889). He later did pioneering work on the physical chemistry of serums, ecology (where he is responsible for much of our early understanding of the greenhouse effect), and cosmology.
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati
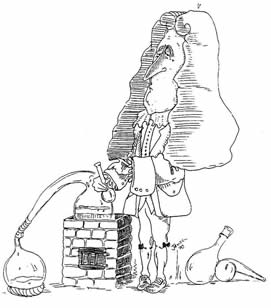
"The Sceptical Chymist"
September 2011
Robert Boyle (1627-1691). Irish chemist and natural philosopher. As a son of the Earl of Cork, Boyle was independently wealthy and so was able to live the life of an independent scientific "virtuoso." Best known for his advocacy of a mechanical corpuscular or particulate approach to physics and chemistry, as opposed to the older hylomorphic doctrines of Aristotle and Paracelsus, Boyle authored numerous books, of which his most famous was perhaps The Sceptical Chymist, first published in 1661. He is known to students of introductory chemistry largely through his discovery of Boyle's law (1662), which states that the volume of a gas varies inversely with applied pressure at constant temperature.
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati

MeasureNet is pleased to announce that its blog will feature caricatures of notable chemists drawn by Professor William Jensen of the University of Cincinnati. The series will include a brief biographical summary of each individual authored by Jensen. The drawings will appear regularly on MeasureNet's its blog beginning in September, 2011.
I'm thrilled to have these Jensen works associated with MeasureNet. They'll add a very nice visual element to our media products. At the same time, they have a high degree of relevance to chemical education. Bill's drawings fit with our efforts to make the study of chemistry more interesting and germane to students of all academic backgrounds.
William B. Jensen, Ph.D. holds the Oesper Chair in the History of Chemistry and Chemical Education at the University of Cincinnati. He is also curator of the Oesper Collection of Rare Books and Portraits in the History of Chemistry and of the department of chemistry's apparatus museum. In the area of the history of chemistry, Dr. Jensen's interests center on the development of late 19th and early 20th century physical chemistry and inorganic chemistry, with special emphasis on the origins of chemical thermodynamics and solid-state inorganic chemistry. He also has made a detailed study of the origins and development of the 19th century scientific community in Cincinnati. Photos of his early 20th-century chemistry laboratory assembled at the University of Cincinnati have been used in MeasureNet brochures and exhibit displays.