Analysis of Vitamin B1 (Thiamine) by Fluorescence Kinetics
Measurement of molecular fluorescence is an important analytical technique in chemical and biological sciences. The capacity of detecting very small fluorophore concentrations, or small changes in its concentration, combined with high specificity make this technique a very powerful analytical tool.
Kinetic methods for determining reaction rates are commonly used. While most experiments are designed to determine the order of a particular reaction in order to gain insight to the reaction mechanism, kinetic methods are also used for quantitative analysis. Determination of the initial reaction rate is one way of quantitatively analyzing a compound within a suitable reaction system.
The following experiment is designed to introduce students to both of these concepts, fluorescence and kinetics as analytical method. This combined technique has the advantage of increased analyte specificity over equilibrium-based fluorescence measurements. As only the compound that is reacting is causing a change in the measured fluorescence signal, steady-state interferences are largely eliminated.
Thiamine (vitamin B1) is essential for the metabolism of carbohydrates and normal function of he nervous and cardiovascular systems. Severe vitamin B1 deficiency will eventually lead to beriberi, characterized by abnormal functions of the muscular and nervous systems, as well as heart and brain abnormalities. Vitamin B1 occurs naturally in foods like whole grains, nuts, vegetables, pork, and liver.
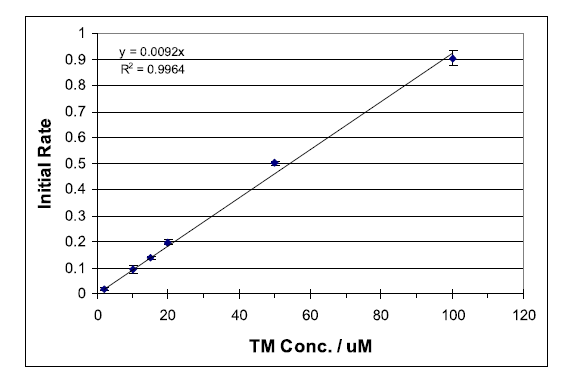
Thiamine (TM), a non-fluorescent compound, has been found to be oxidized selectively by mercuric oxide (HgO) at a rate suitable for monitoring with standard fluorescence spectrometers.1 The oxidation product, thiochrome (TC), is fluorescent with a strong absorbance maximum at 367 nm and fluorescence emission at 444 nm. Deprotonation of TM yields a non-fluorescent tricyclic intermediate (CI), which is oxidized to TC as outlined in Scheme 1. Immediate oxidation of CI is essential to avoid the formation of several non-fluorescent productions.1 Thus, it is important to follow the sequential addition of reactants as outlined below... To view the complete experiment Click Here.