Svante Arrhenius (1859-1927). Swedish Chemist. Lecturer and Professor at the Technical Institute in Stockholm (1891-1904). Nobel Prize Winner (1903) and later Director of the Nobel Institute (1905-1927). Arrhenius is best known for his theory of ionic dissociation, which evolved out of his doctoral thesis of 1884, his ionic acid-based definitions (1887), and his introduction of the concept of activation energy in chemical kinetics (1889). He later did pioneering work on the physical chemistry of serums, ecology (where he is responsible for much of our early understanding of the greenhouse effect), and cosmology.
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati

"Gas"
Joseph Priestley (1733-1804). British chemist and Unitarian Minister. Priestley earned his living as a minister and teacher at various dissenting academies. His work in chemistry was done in his spare time and largely during the period when he served as private librarian to Lord Shelburne. It dealt almost exclusively with the use of the pneumatic trough to discover a large number of new "airs" or gases, including nitrogen oxide, nitrogen dioxide, dinitrogen oxide, ammonia, hydrogen chloride, sulfur dioxide and, most famously of all, oxygen (1774). He also investigated the processes of brewing, photosynthesis, respiration, and invented soda water. Priestley was a prolific writer, not only on chemistry, but also on the subjects of theology, history, geography, natural philosophy, and electricity. Due to
his liberal political opinions, his home and laboratory were destroyed by a Birmingham mob in 1791, and he spent his final years in Northumberland Pennsylvania, where he wrote several pamphlets defending the outdated phlogiston theory against Lavoisier's newer oxygen theory of combustion.
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati
 LUMINESCENCE/PHOSPHORIMETRY
LUMINESCENCE/PHOSPHORIMETRY
Last, but certainly not least, is the use of the MeasureNet “Colorimeter” as a “Phosphorimeter”… also called a “Luminometer”… which, is one of the more versatile Analytical “tools” not readily found in most laboratories. A “Phosphorecent” response can be induced by UV-fluorescence AFTER the light souce is removed… or it can be caused by a chemical reaction that causes the “emission” of VISIBLE Light photons. The proprietary design of the “Colorimeter” optical layout allows the analyst to get the desired response from their sample material… again using the same high-sensitivity optics.
Bio-Luminescence in Fish and Algae, Luciferin/Luciferase Reaction in Fireflies, UV-Blocking capacity of SPF Lotions, the ‘controllable' oxidation of Luminol Light-Sticks, the newly developed photo-luminescent additives for plastics used in skyscraper building and more are beginning labs you can use directly as-is, or modify for your own specific curriculum… as outlined for your review:
Luminescence (“Phosphorescence”) for Kinetic & Relative Comparison values:
• Check out these Glow-in-the-Dark Plastics… some are made of material quite Fantastic!
• Bio-Luminescent Algae and Bacteria are found in “Healthy” Waters… See anything “Fishy”?
• P-AminoBenzoic Acid is the SPF of “Old”… using Luminscence its tale can be told!
• “UV” Light causes “Glow-in-the-Dark” Photon Motions… “Block” it with some SPF Lotion!
• “Luciferase” is the enzyme in the Fire-Fly… catch a few & give this Phosphorimetry Lab a try!
• The Luminol “Light Stick” reaction… uses Organic Oxidation to set those Photons in action!
Many current MeasureNet users that have the MDBC-138 “Colorimeter”… and potential customers, too… may not be aware of the tremendous versatility of this spectral device… and many potential *new* Customers in academia can save a significant amount of time, money and resources by implementing this technology for their teaching laboratories and educational curricula. Designed for simple, direct plug-n-play capability with any MeasureNet Workstation.
The Princeton Section of the American Chemical Society will be holding their annual National Chemistry Week Activities Night On Friday evening, October 21 from 7-9 pm. It will be held at Princeton University, Frick Laboratory, located on Washington Road, Princeton NJ. This year's theme is "Chemistry - Our Health, Our Future!
A week of fun and educational activities are planned in
Manhattan to showcase how chemistry makes life better. The events are part of National Chemistry Week, Oct. 16-22, and are sponsored by Kansas State University's local section of the American Chemical Society and the department of chemistry.
We will celebrate IYC 2011 at our yearly NCW Event held at the NY Hall of Science on Sat., 10/22! Local universities and companies will have a variety of cool hands-on demos for kids ages 5-15! We will also host a "Chemistry Bingo" where winners will win an IYC 2011 lapel pin and other neat goodies!
Enjoy classic chemistry experiments and learn the positive impacts of chemistry as it relates to nutrition, hygiene, and medicine.
10:00 - Chemistry activities, Protozone - Level 1
11:30 - Chemistry activities, Protozone - Level 1
1:00 - Cool Chemistry Program, Demonstration Station - Level 1
The No. Jersey Section's NCW celebration and student poster session will take place at a
ChemExpo celebration at Liberty Science Center on
Saturday, October 22 from 10:00 AM to 2:00 PM.
Saturday, Oct 22 10:00a to 1:00p
The Santa Clara Valley section of the American Chemical Society will hold a National Chemistry Week Celebration for kids of all ages. The Wheel of Chemistry Fortune will be spinning for all kids to win a prize, and there will be fun hands-on chemistry including a slime lab! This will also be an opportunity to pick up a free copy of “Celebrating Chemistry”, the NCW newsletter for elementary-aged children.
The New York Section of the American Chemical Society will be celebrating National Chemistry Week on October 22, 2011 at the Great Hall in the New York Hall of Science. This day-long event will showcase chemistry principles using demonstrations performed by local college students and volunteers from local industries for children of all ages. This year's theme is "Behind the Scenes with Chemistry" and the event will run from 11 am to 4 pm.
As part of the National Chemistry Week 2011 celebration, the International Year of Chemistry (IYC) and in recognition of its theme, "Chemistry - Our Health, Our Future!" The American Chemical Society (ACS) and the Department of Chemistry at Texas A&M University are sponsoring an illustrated poem contest for students living in the Brazos, Robertson, Burleson, Washington, Grimes, Madison, and Lean Counties in grades K-12.
National Chemistry Week, sponsored by the American Chemical Society, is celebrated at Gordon College by our department. See some of the different ways students and faculty get involved.
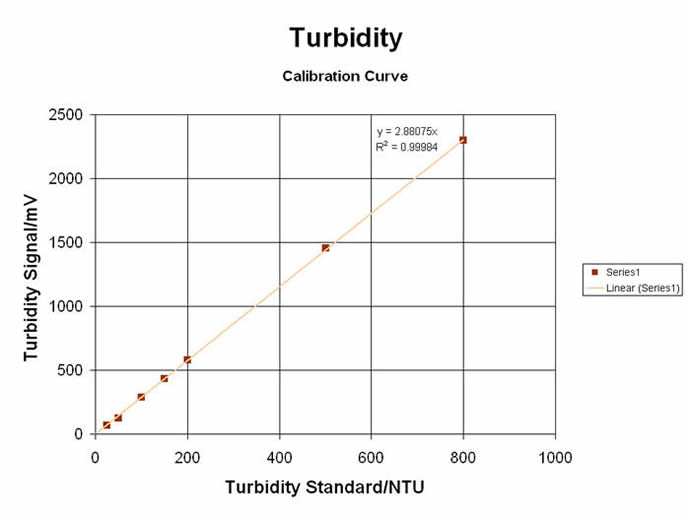
#3) TURBIDIMETRIC / NEPHELOMETRIC LIGHT-SCATTERING SPECTROMETRY
The “Colorimeter” can also be used in the “Turbidometer” mode… or more precisely, as a “Nephelometer”, since nephelometry uses a Near-IR LED (@ 880nm) to measure the SCATTERED light at a 90° angle from the light source, as it is done on the MeasureNet unit. Tests can be made on sample solutions that have existing particulate suspensions… or a reaction can be made to produce a range of particulates for relative and direct measurements. Originally used for Clinical Chemistry applications, Turbidometry/Nephelometry can be applied to a wide range of curriculum subjects… from Organic Synthesis & Analytical Chemistry to Environmental & Biochemistry Labs. Some of these unique and educational labs are shown here:
Turbidity (“Nephelometry”) for Kinetic & Relative Comparison at Single values:
• Environmental Testing of “Settleable Solids” for River / Stream & Estuarial Waters
• Reducing Sugars by Benedict’s Test... great for Diabetics, Atkins-Diets and all the rest!
• Collect some of the DUST from your “Air”… is it okay to breathe or is something there?
• Rate of Reaction of Silver Ion + Chloride Ion to form AgCl precipitate… Kool Kinetics!
• The classic Princeton “Nassau Clock” Reaction… makes for a great teaching Lab attraction!
• Measurement of Yeast Growth in Beer… is it fermenting slow or in high-gear?
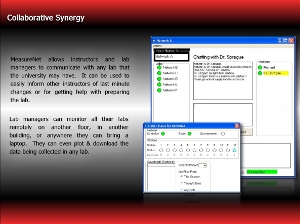
Have you ever wanted to look in from your office or remote location to check on the progress of your lab students or an experiment you are running? Or what about sharing experiment data from this morning’s lab with rural student miles away with no lab access? With MeasureNet’s new Remote Monitor software, you can— whether you’re down the hall or across the country.
Designed with STEM partnerships and outreach activities in mind, Remote Monitor gives institutions without MeasureNet a chance to participate through the graphical viewing of acquired data files and the analysis of collected data via most any statistical or spreadsheet software. It also allows lab directors, instructors, and teaching assistants to monitor their MeasureNet networks from different physical locations.
Any station on any network can be monitored live or its saved files can be downloaded to any PC running Remote Monitor. Network chat boxes make it easy for teaching assistants to communicate with each other or with the lab manager. Remote Monitor also enables MeasureNet’s Cincinnati offices to quickly troubleshoot a network anywhere in the world as long as it is connected to the Internet. With appropriate IDs and passwords, a collaborating institution can have access to partner real-time lab experiments or saved data for research or outreach activities. Remote Monitor installs on any Windows-based PC connected to the Internet and doesn't require additional software or MeasureNet hardware.
MeasureNet Technology Ltd. manufactures patented, network-based data acquisition interfaces for science teaching laboratories. It is a spin-off of the University of Cincinnati's Department of Chemistry and is headquartered in Cincinnati, Ohio. Measurenet's award-winning, PC-reducing design helps reduce laboratory maintenance and operational costs while giving students access to high quality shared UV-vis spectroscopy, gas chromatograph connectivity, and an array of innovative probeware. Its acclaimed intuitive design provides improved transparency to enable better science-focused, not technology-focused, learning. Winner of the Ohio Governor's Award For Excellence in Energy Efficiency, MeasureNet networks are found in universities, community colleges, high schools, and vocational training centers across the United States and around the world.
 On Sept. 26 the U.S. Department of Education announced that 97 Hispanic-Serving Institutions (HSIs) are the recipients of awards given through the Hispanic-Serving Institutions Science, Technology, Engineering and Mathematics, and Articulation Programs (HSI STEM and Articulation Programs). The program had about $100 million for 109 grants that will support the development of articulation between two and four year institutions or enhance science, technology, engineering and math (STEM) programs at HSIs. The grant funds may be used for:
On Sept. 26 the U.S. Department of Education announced that 97 Hispanic-Serving Institutions (HSIs) are the recipients of awards given through the Hispanic-Serving Institutions Science, Technology, Engineering and Mathematics, and Articulation Programs (HSI STEM and Articulation Programs). The program had about $100 million for 109 grants that will support the development of articulation between two and four year institutions or enhance science, technology, engineering and math (STEM) programs at HSIs. The grant funds may be used for:
- scientific or laboratory equipment for teaching
- the construction or renovation of facilities
- purchasing educational materials
- academic tutoring or counseling programs
- teacher education
- student support services.
More....
QUANTUM FLUORESCENCE & KINETIC SPECTROMETRY
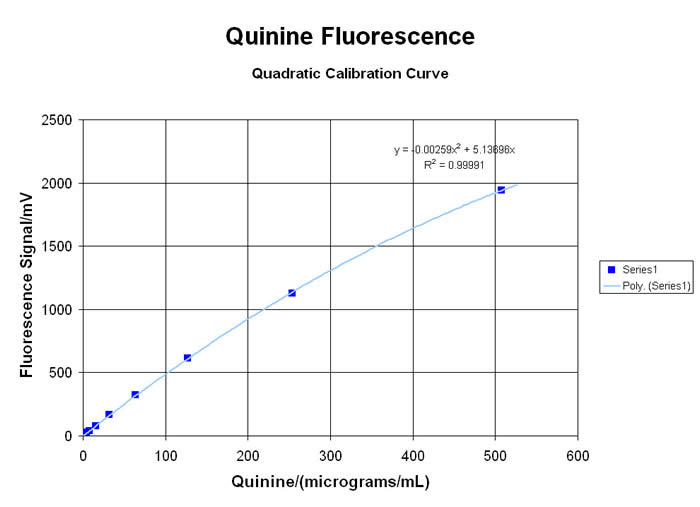
Many types of organic molecules will exhibit a VISIBLE fluorescent “emission” when exposed to high energy ULTRA-VIOLET light to create the “excitation”. This spectro-chemical response is due to the interaction of the energetic UV photons with the loosely-held pi-electrons and other “labile” functional groups in aromatic, olefinic, alkaloidal, xanthinoid and poly-cyclic compounds… and there are many important applications for this little-understood, rarely-taught, under-utilized technology. Using the newest in LED technology, the MeasureNet “Colorimeter” has several “excitation” sources available… with the 375nm UV-LED providing the most useful range of responses for relative fluorescence analyses (since it is very close to the classic 366nm long-wavelength UV from a mercury lamp)… although several alternate user-defined Wavelengths are available from a growing list of LED sources. Please inquire for more information!
When used in the “Fluorometer” mode, the detector is situated at a 90° angle from the UV LED Source, to generate TRUE fluorescent “emission” data without potential interference from the “excitation” wavelength. Laboratory exercises for both qualitative comparisons and quantitative analysis of many materials are possible with the MDBC-138 Dual-Beam Colorimeter. Some applications in Organic Chemistry, Analytical Sciences, Biology/Biochemistry, Environmental and Nutritional Science programs, are highlighted here:
UV-LED Fluorometry for Single-range EMISSION Values:
• “Zap” UV against Chlorophyll from things that Grow… and see what makes it Glow
• The Highlights of Highlighting Markers: How bright is bright?
• Evaluation of Cigarette Second-Hand Smoke: Nicotine for the Masses in your Breathing Gases?
• Analysis of Alkaloid Materials like Quinine
• Measure Amino Acids in “Energy Drinks”… Are their labels Accurate
• Check Ground-Water “Plumes” with Fluoresceine… Water-Table extracts will also be green!
A typical colorimeter is usually a simple, single-beam optical system to measure “color” in the VISIBLE Spectrum of light and provide ABSORBANCE data at a single wavelength. These systems can cost $1,000 or more for stand-alone colorimeters that merely provide simple ABS data… unless you consider the MeasureNet Model MDBC-138 true Double-Beam, Multi-Functional “Colorimeter” based on a unique set of LEDs (Light-Emitting Diodes)... for under $500! The MeasureNet “Colorimeter” is actually FOUR (4) instruments in ONE (1) compact and rugged box… and works as a colorimeter (to make Beer’s Law Curves for VISIBLE Wavelengths), a UV fluorometer (to demonstrate fluorescence & quenching in certain organic molecules), a turbidometer (to measure the turbidity of particulate & colloidal suspensions) and a phosphorimeter (to analyze the phosphorescence [“glow”] of specific materials).
#1) BEER’S LAW ABSORBANCE & KINETIC SPECTROSCOPY
This unit can be used as a “simple” Colorimeter to demonstrate Beer’s Law using three (3) high-output VISIBLE Light LEDs at 472nm (BLUE), 525nm (GREEN) and 630nm (RED); which cover over 75% of the classic General Chemistry and Analytical Chemistry Laboratory experiments that teach spectrophotometric measurements. The wavelength coverage of these LEDs allows highly accurate relative measurement of almost all the ROYGBIV Colors. The disposable & unbreakable 10mm pathlength, near-UV plastic cuvettes included with the “Colorimeter” can be used from ~300nm (in the near-UV) to over 1000nm (in the near-IR) for analytical measurements… and come with sealing caps to preserve prepared solutions for future tests. Some of the popular Laboratory experiments for your Academic Lab curriculum programs Gen Chem, Analytical or Student Research are:
Red / Blue / Green Colorimetric ABS Data for Beer’s Law plots:
• Test for Mineral IRON in your food… check your Cereals, Breads and more
• Unsaturated FATS can be easily seen… just get a purple Color using some IODINE!
• Evaluation of Nutritional Food Proteins… React it to get a “BLUE” and see what can be seen!
• Consistency of M&M and Skittles Candy Colors: Is the "blue" true blue or “faux” to you!
• Changing “Colors” of some pH Dyes… [H+] makes them look *new* to our eyes!
• General Colorimetric Assay… for ANION (X, PO4, SO4, NO3, NO2, etc)
The MDBC-138 is a TRUE Double-Beam Optical System… and provides a reference cell to “blank” out the reagents used to create a very stable ABS reading for several Organic Reactions. Data from these Labs can be used to calculate rate constants, equilibrium factors and reaction conditions (for thermal, ionic and electrochemical variables). This is a MUST for making accurate kinetic experiment tests. A few of them from our “Library” include:
Dual-Beam ABS Data for Kinetic Measurements:
• The Iodine Test for Starches… just Hydrolyze with Amylaze to get a Kinetic Rate
• Perform REDOX “Clock” Reactions
• pH-based Hydrolysis of p-nitrophenylacetate Ester

What makes the MeasureNet temperature probe unique? Two features in combination are what set this probe apart from other stainless-steel sheathed probes on the market; its rapid four-second response time and its acid resistant coating. These two characteristics are essential for conducting experiments like thermometric titrations and freezing point depressions in the general chemistry lab.
Before I go into the details of how we achieve these two essential characteristics, lets examine the most common temperature sensor options found in the chemistry lab. Listed in the table below are the three types most commonly used sensors and their properties:
| Attribute |
Thermocouple |
RTD |
Thermistor |
| Cost |
Low |
High |
Low |
| Temperature Range |
Very wide
-350oF
+3200oF |
Wide
-400oF
+1200oF |
Short to medium
-100oF
+500oF |
| Interchange ability |
Good |
Excellent |
Poor to fair |
| Long-term Stability |
Poor to fair |
Good |
Poor |
| Accuracy |
Medium |
High |
Medium |
| Repeatability |
Poor to fair |
Excellent |
Fair to good |
| Sensitivity (output) |
Low |
Medium |
Very high |
| Response |
Medium to fast |
Medium |
Medium to fast |
| Linearity |
Fair |
Good |
Poor |
| Self Heating |
No |
Very low to low |
High |
| Point (end) Sensitive |
Excellent |
Fair |
Good |
| Lead Effect |
High |
Medium |
Low |
| Size/Packaging |
Small to large |
Medium to small |
Small to medium |
(Table is courtesy of The Enginneering ToolBox)
One sensor not mentioned is the solid-state temperature sensor. We selected the LM35CH solid-state sensor for the MeasureNet temperature probe for three reasons; its response time, temperature range, and its easy interface to the measurement stations.
In designing the MeasureNet temperature probe, we focused on two characteristics; response time and acid resistance. The response time is particularly pertinent when selecting a temperature probe. When trying to measure a fast thermal reaction with a temperature probe that has a slow response time, as they say, garbage-in equals garbage-out! The other consideration is acid resistance, which impacts the probes durability.
This is where MeasureNet's acid resistant and thermally conductive coating comes in to play. It maintains its superior protective properties over long periods of time with minimal impact on its response time. It has passed extensive tests for protecting the probe in hydrochloric, phosphoric, sulfuric and nitric acid solutions. Most of the temperature probes on the market designed for rugged lab use embed the temperature sensor in a stainless-steel sheath. Although this gives the probe superior mechanical strength and some protection against weak acids, it slows the response time of the probe to 8-30 seconds depending on the sensor used and the design of the sheath. MeasureNet designed the temperature probe with the end user in mind, students in the chemistry lab.