
MeasureNet's ElectroJelly™ pH electrode has been designed with students and the chemistry teaching lab in mind. What is ElectroJelly™? It is a solid material with highly concentrated salt (most times it is KCl) that can effectively hold reference electrode electrolytes and prevent it from contamination by sample back-flow. The ElectroJelly™ filled (sealed) electrodes have a longer life and need less calibration than regular gel filled electrodes, saving time and money. Two other features unique to the MeasureNet pH probe are the SilverCap™ for noise reduction while handling the probe, and the epoxy body design uses holes instead of tabs too better protect the glass bulb from the rough handling of students. You use the pH probe with our Drop Counter for pH titrations.
ElectroJelly™ pH Electrode Features:
- Superior Stability
- Fast response
- Minimum sodium (alkaline) error
- pH 0 -14 full range measurement
- Special internal fill offers full range linear temperature compensation
- Very low sensor glass membrane resistance
- Zero and Isopotential: ~pH 7
- Unique Glass Bulb protection design
MeasureNet's ElectroJelly™ probes are ideal for undergraduate research projects. The probe's stability and long life span are ideal for 24/7 monitoring applications and experiments involving temperature cycling. If pH titrations are an fundamental part of your curriculum or research, the ElectroJelly™ probes stability and rapid response time makes it the perfect choice.
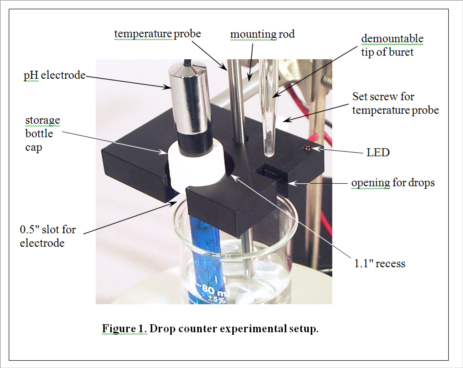
Electronic Measurement and Data Collection in the Chemistry Laboratory
Hands-on Experiment - pH Titration Curve
In this experiment, you will generate a titration curve for an unknown acid or base. Using a drop counter, you will add titrant and display a plot of pH vs. drops on the MeasureNet™ workstation.
Procedure
Calibrating the pH electrode
1. Move the black band on the pH electrode to uncover the hole in its side. Mount the electrode in a vertical position with a universal clamp on a ring stand (not the same one holding the burets), and connect it to the workstation. Place a waste beaker under the electrode and rinse the electrode with distilled water, then gently touch a Kimwipe to the electrode tip to remove the remaining drop of water. Pour a sample of a standard buffer solution into a disposable cup. Lower the pH electrode into the solution.
2. Press MAIN MENU on the workstation. A list of measurement types will appear on the screen. (Make a note of the station number listed at the top of the screen.) Press the function key listed for pH, then the function key for pH versus drops, and then press CALIBRATE. Follow the instructions on the screen, which will prompt you first to enter the temperature (if not known, just assume ~22°C), then to enter the known pH of the standard solution, and finally to monitor the pH displayed. Be sure to press Enter after the pH reading becomes steady. You are performing a single point calibration, so press F1 when prompted by the message on the workstation screen. Press DISPLAY and note the pH value on the workstation screen. If it is not within 0.02 pH units of the actual pH of the standard buffer solution, you should redo the calibration.
Titration Experiment
(Consult Figure 1 on the last page for a picture of the experimental setup.)
3. Select an unknown solution, which will contain either a mono- or diprotic weak acid, or a mono- or dibasic weak base. Pipet 25 mL of the unknown solution into a 50-mL beaker equipped with a stir bar. Place the beaker on the center of the stir plate. Place the pH electrode in the holder in the drop counter, and lower it into the beaker (near the wall of the beaker to avoid the stir bar) so that the protective shield rests lightly on the bottom of the beaker. Clamp the drop counter to a ring stand. Turn on the stir plate so that the stir bar is turning at the lowest sustainable speed. Note the initial pH of the solution. Based on this pH, decide which titrant (HCl or NaOH) you will use to titrate the solution. Fill a 50 mL buret with the appropriate titrant.
4. Position the buret containing the selected titrant so that its tip is over the appropriate opening in the drop counter, making sure that this is also over the beaker, probably near the wall opposite the pH electrode. It gets a little crowded here, and you will need to take care not to change the position of the beaker in the center of the stir plate. Don't let any titrant fall into the sample yet! When everything is arranged as in Figure 1 (except the temperature probe, which we aren’t using in this experiment), begin the titration by pressing START/STOP on the workstation. The station will first ask you to enter the starting reading on the buret. It need not be exactly 0.00 mL. Just read what it is and enter the appropriate value. The station display will now show the pH and the number of drops counted, 0 so far. Carefully turn the stopcock on the buret to begin adding the titrant. The red LED on the drop counter will flash each time a drop is counted. Try to maintain a rate of approximately 1 drop per second. A flow restrictor is used in the stopcock to make this easier to accomplish. Your only duty while the titration is running is to monitor the drip rate and make small adjustments of the stopcock if needed.
5. When you have followed the titration far enough, press START/STOP to stop the titration. The station will now ask you for the final volume reading on the buret. Read and enter the value.
6. Print your titration curve. Press FILE OPTIONS, read the menu that appears, and press the function key listed for PRINT DATA (standard). Enter the number of copies to be printed, and press ENTER. Your plots will appear on the printer attached to the PC. They will be labeled at the top with your station number.
7. Use the printed plot to estimate the pKa(s) for the substance you titrated, then ask the instructor to compare your results with what was expected for your unknown.
"The Sceptical Chymist"
September 2011
Robert Boyle (1627-1691). Irish chemist and natural philosopher. As a son of the Earl of Cork, Boyle was independently wealthy and so was able to live the life of an independent scientific "virtuoso." Best known for his advocacy of a mechanical corpuscular or particulate approach to physics and chemistry, as opposed to the older hylomorphic doctrines of Aristotle and Paracelsus, Boyle authored numerous books, of which his most famous was perhaps The Sceptical Chymist, first published in 1661. He is known to students of introductory chemistry largely through his discovery of Boyle's law (1662), which states that the volume of a gas varies inversely with applied pressure at constant temperature.
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati

The American Chemical Society has been awarded a $1 million chemistry public outreach grant from NSF. According to C&EN editor Sophia L. Rovner, the grant will be used to communicate the contributions of chemistry to society. This chemical education grant from NSF is timed perfectly because, 2011 is the International Year of Chemistry.
The grant "enables ACS efforts to sustain the momentum of IYC 2011," says ACS Executive Director and CEO Madeleine Jacobs . "Our members, committees, local sections, technical divisions, and our external IYC Partners—and their networks—will be vital to help the project be a success."
To learn more go to C&EN article ACS Wins $1 Million NSF Grant.

MeasureNet is pleased to announce that its blog will feature caricatures of notable chemists drawn by Professor William Jensen of the University of Cincinnati. The series will include a brief biographical summary of each individual authored by Jensen. The drawings will appear regularly on MeasureNet's its blog beginning in September, 2011.
I'm thrilled to have these Jensen works associated with MeasureNet. They'll add a very nice visual element to our media products. At the same time, they have a high degree of relevance to chemical education. Bill's drawings fit with our efforts to make the study of chemistry more interesting and germane to students of all academic backgrounds.
William B. Jensen, Ph.D. holds the Oesper Chair in the History of Chemistry and Chemical Education at the University of Cincinnati. He is also curator of the Oesper Collection of Rare Books and Portraits in the History of Chemistry and of the department of chemistry's apparatus museum. In the area of the history of chemistry, Dr. Jensen's interests center on the development of late 19th and early 20th century physical chemistry and inorganic chemistry, with special emphasis on the origins of chemical thermodynamics and solid-state inorganic chemistry. He also has made a detailed study of the origins and development of the 19th century scientific community in Cincinnati. Photos of his early 20th-century chemistry laboratory assembled at the University of Cincinnati have been used in MeasureNet brochures and exhibit displays.
The 242nd American Chemical Society is approaching fast. This year the conference will be held on August 29 - August 31st in Denver.  The theme of this years conference is " The Chemistry of Air, Space and water". Ironically the theme fits the current heat and drought conditions plaging many areas of the US and the sad ending of the space shuttle program.
The theme of this years conference is " The Chemistry of Air, Space and water". Ironically the theme fits the current heat and drought conditions plaging many areas of the US and the sad ending of the space shuttle program.
Here is a breif list of some of the more interesting programs at the conference.
Foods for Extreme Environments
Modern Agriculture and Biotechnology – Tools for Sustainability
Chemistry in Mobile Spaces: Chemical Apps for Mobile Devices:
Novel Solutions to Water Pollution
Urban Greehouse Gas Emissions, Climate Change, and Mitigating Impacts
Irv Levy, The Division of Chemical Education program chair has created a great program line up for this years conference.
Greening Undergraduate Education: Lecture and Laboratory Innovations
Jerry Bell and the Joy of Chemistry
Celebrating IYC 2011 through Education and Community Outreach
Research at Community Colleges: Strategies for Enhancing Student Transfer and Success
Creating Innovation by Collaboration in Green Chemistry Between Industry University Centers and Students
Chemistry on Stamps Exhibition
For a complete program list - 242nd ACS Program.
Please make sure to take some time to visit the Vendor exposition. MeasureNet will be at booth 1505, Mark Hoffman will be manning the booth this year. Mark will be happy to tell you about some of the new products MeasureNet has introduced this year. Here are of some the exciting new additions to our Multifunctional Chemical Analysis Network ( MCAN ) technology for chemistry lab.
Cloud based Student Lab Report feature has been added to student data storage site - LabLinx. Students will now be able to submit their lab reports online saving time and paper. We have also a Click and View capablity so students can instantly view their laboratory data without having to download and import into Excel to view their data, another big time saver.
For upper level courses we have added a cloud based experiment remote monitor and alert feature. This allows students and researches to monitor their experiments over the internet with computers or smart phones. It even has a text alert feature to let you know if something has gone wrong. We will be adding many more features to this remote monitor capability in the coming months.
We look forward to another great conference in Denver and hope to see you there.
 A Journal of Chemical Education article titled Managing Laboratory Data Using Cloud Computing as an Organizational Tool by Jacqueline Bennett and Harry E. Pence (J. Chem. Educ. June 2011:Vol. 88 no. 8) brings to light the the key role cloud computing will play in laboratory instruction.
A Journal of Chemical Education article titled Managing Laboratory Data Using Cloud Computing as an Organizational Tool by Jacqueline Bennett and Harry E. Pence (J. Chem. Educ. June 2011:Vol. 88 no. 8) brings to light the the key role cloud computing will play in laboratory instruction.
The authors summarized the benefits of the cloud from a research and educational perspective nicely... "Cloud computing, where both software and computer files reside online, offers a solution to this data-management problem and allows researchers to coordinate their efforts just as easily whether they are working in the same laboratory or laboratories halfway around the world ".
MeasureNet Technology has been supplying chemistry lab instructors with cloud based tools for data-management, data security and Collaborative/Cooperative Learning solutions for many years. MeasureNet's Laboratory Electronic Measurement and Data Collection Technology is used in university chemistry labs by students to measure and collect data sets in the lab, store and share on the cloud and analyze these data sets from anywhere via the cloud. MeasureNet's patented unique Network design takes cloud computing to the next level by connecting students to the lab TA or instructor while they are are conducting the experiments. Using MeasureNet's real-time monitoring capablities, TA's in the lab can monitor student experiments to make sure they are conducting the experiments properly. Instructors or collaborators can also monitor the live data collection with their computers from any remote location via the internet.