
"Annalen"
Justus von Liebig (1803-1873) Professor at Giessen (1824-1852) and Munich (1852-1873), Liebig's teaching laboratory at Giessen served as the model for the advanced training of chemists in the first half of the 19th century, including many foreign students from Great Britain and the United States, and, through his editorship (starting in 1832) of the journal, Annalen der Chemie und der Pharmacie (which is still published under the name of Liebig's Annalen), he exercised enormous influence on the early development of organic chemistry, agricultural chemistry, and physiology. He perfected organic combustion analysis (1837) and the counter-current laboratory condenser (1843), and, in collaboration with his close friend, Friedrich Wöhler, did pioneering work on the isomerism of fulminates (1823-1826) and the reactivity of the benzoyl radical (1832).
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati

Carl Sagan once said, “It’s suicidal to create a society that depends on science and technology in which no one knows anything about science and technology.”
The United States is slipping behind other countries in awarding postsecondary STEM degrees. Between 1998 and 2006, the total number of STEM degrees grew by 23 percent in the United States. During this same period, Poland increased its number of STEM degrees by 144 percent and Taiwan boasted a 178 percent increase. China’s number of postsecondary STEM degrees exploded by a stunning 200 percent. In 2006, China awarded nearly double the number of postsecondary STEM degrees gained in the United States. The USA needs more STEM degrees if it hopes to compete in tomorrow’s global economy.
More STEM degrees mean more STEM jobs. Increasing the number of jobs in STEM fields is critical to the economic prosperity of both individuals and communities. Generally speaking, STEM jobs are some of the highest paying positions, with wages significantly above the US average. STEM jobs represent one of the fastest-growing segments of the job market, both in the United States and globally. Between 2008 and 2018, the number of STEM jobs is expected to have grown by 17 percent.
STEM occupations are associated with lower unemployment rates when compared to other professions. Students who graduate with a STEM degree but pursue jobs in non-STEM fields also make more money than those with degrees in other fields. Cities across the nation try to attract STEM professionals because civic leaders understand that the high pay and economic stability associated with STEM jobs benefits the economic and social fabric of the community.
The main pathway to a lucrative career in STEM fields is through postsecondary education. Getting bright minds into postsecondary programs poses a two-fold challenge to secondary educators – sparking interest in pursuing further education in STEM studies and properly preparing students to engage in higher learning. In most K–12 systems today, math and science subjects seem to have little to do with the real world. Students often ask, “When will I ever use this knowledge?” Students just don’t seem interested in STEM and educational institutions fail those few students leaning towards a STEM degree.
The National Governors Association explored this challenge in the December 2011 updated version of Building a Science, Technology, Engineering and Math Education Agenda. This committee examined the goals of the STEM agenda and outlined why this agenda is so important to the states and to the nation. They identified weak links in the system and outlined strategies to implement state-wide STEM agendas in ways that excite students about STEM studies and careers while giving them the tools to succeed in gaining a degree and securing a job.
The STEM agenda has two basic goals: expand the number of students entering postsecondary STEM studies and increase STEM proficiency in the general student population. The first goal improves the technical capabilities of the nation’s workforce while the second goal helps students implement concepts and problem-solving strategies gained through STEM studies into their everyday lives.
Proficiency in STEM facts, principles and techniques are just as beneficial to future employees of big-box stores as they are to the physicians and engineers of tomorrow. Integrating STEM concepts and hands-on learning into the regular curriculum will help the general student population hone critical thinking skills to recognize, evaluate and solve problems not related to science, technology, engineering or mathematics.
This study identified five areas that states are trying to improve upon in an effort to increase the number of interested students qualified to pursue postsecondary STEM studies. Inconsistent state standards, a shortfall of qualified instructors and failure to motivate student interest all prevent students from pursuing postsecondary studies in STEM. This study also suggests that students are not prepared for postsecondary STEM study because they lack hands-on learning and experience with laboratory equipment. Worse yet, many current postsecondary STEM studies do not properly prepare students to work in STEM fields.
Fortunately, the report by the National Governors Association contains solid research that provides direction to increasing the presence of STEM tools in your learning laboratory in a way that propels your students into postsecondary studies and high paying STEM jobs. Building a Science, Technology, Engineering and Math Education Agenda says that several studies correlate strong preparation in high school with improved STEM degree completion rates. Furthermore, certain high school instructional practices seem to be more effective than others, including doing hands-on experiments in science. Encouraging high school students to form workgroups also improves postsecondary STEM outcomes.
Use informal learning to help students make the connection between STEM classes and real-world applications. Field trips to museums, science centers and other public and private institutions showcase job opportunities for those with degrees in STEM fields. Many of these institutions provide hands-on activities using the same equipment professionals employ. The connections between STEM classes and real-world applications are reinforced when your students can then use these same pieces of equipment in their own classroom laboratories.
The National Governors Association notes that a “student’s ability to enter and complete a STEM postsecondary degree or credential is often jeopardized because the pupil did not take sufficiently challenging courses in high school or spend enough time practicing STEM skills in hands-on activities.” Hands-on learning chemistry labs improve a student’s experience in secondary school, giving her confidence to succeed in postsecondary STEM studies or in an entry-level non-STEM career.
Our nation’s security and economic stability rest in the capable hands of exceptional educators just like you. It is up to STEM educators to expand the knowledge of science and technology not only in collegiate hopefuls but in the student population as a whole. Improve your students’ chances of success competing in a global economy by training them the same chemistry laboratory equipment that universities and professionals use.
Science and technology bond together like carbon and hydrogen. One field benefits the other- scientific discoveries advance technological applications which then return more sophisticated research tools to the scientific community. Many of your chemistry students will graduate into a world that integrates science and technology into a singular platform that utilizes electronic data collection technology, a safer and more efficient mode of gathering and managing information. Give your freshman chemistry students the technological edge they need to facilitate learning while simultaneously freeing yourself from expensive hardware upgrades, viruses and archaic forms of monitoring student progress in your freshman chemistry lab.
When you switch to electronic data collection technology, you’ll immediately notice how much more available space you have in your general chemistry lab. In the typical, old-fashioned general chemistry lab, each student shared bench space with their own large, cumbersome PC. These PCs are at increased risk for virus infection, costly hardware and software upgrades and usually need to be replaced every three to four years. The MeasureNet MCAN, or Multifunctional Chemical Analysis Network, replaces up to 15 individual PCs with a single PC that is used by the instructor to monitor student activity and manage their data files, locally or on the cloud.
Student workstations integrate with a wide variety of probes and other chemistry laboratory apparatus that enable your chemistry lab students to accurately perform hands-on experiments in general chemistry, environmental chemistry, STEM and biochemistry labs. These hands-on lab exercises are critical for the students’ development of basic chemistry concepts.
Chances are good that students in your general chemistry lab are already technologically advanced and have used electronic data collection technology in high school. They are also quite accustomed to cloud computing from using products like Google Docs and Dropbox, where both software and files are saved online rather than on a personal computer. MeasureNet’s MCAN technology merges electronic data collection and cloud computing capabilities together. MCAN allows your student to measure and collect high resolution data in the lab and store it on the cloud for later analysis and lab report generation. Students, especially science students, will be excited to use the advanced technology MeasureNet MCAN offers. MeasureNet MCAN technology takes your students to the next level, giving your students the edge they need when whether they go on to industry or pursue advanced degrees in science.
Cloud computing also helps you monitor your chemistry lab students from the instructors PC to be sure they are conducting the experiment, collecting data and analyzing information properly. You can also monitor live data collection experiments remotely. Using the internet you can connect to MeasureNet MCAN workstations from outside the chemistry lab using a computer, tablet or smart phone.
MCAN electronic data collection technology can easily be in integrated into your current chemistry lab curriculum and can be adapted to fit a variety of teaching styles.
- POGIL
- STEM
- Self Directed
- Verification-style
- Inquiry-based Chemistry Labs
- Hands-on learning
You can easily integrate MeasureNet based experiments into your current lab curriculum. Here is a small sample of experiments that can be conducted with the MCAN technology.
- Gas Laws
- Colligative Properties
- Enthalpy of Reaction — Hess's Law
- Determination of the Heat of Neutralization of a Variety of Strong Acids and Bases
- Chemical Kinetics
- Determination of a Reaction Equalibrium Constant Using Absorption Spectroscopy
- pH and Buffer Solutions
- pH titrations and end-point determination using Drop Counter
- Identifying a Weak Unknown Acid
- Determination of the Molecular Weight of a Volatile Liquid Using the Ideal Gas Law
- Vapor Pressure and Heat of Vaporization
Using MeasureNet’s MCAN technology in your lab means you’ll spend less time working as a computer repair technician and more time teaching chemistry. The MeasureNet MCAN frees you from problems usually associated with PC-based systems. Virus removal and reimaging computers will be a thing of the past.
Get back to doing what you love – teaching chemistry to hungry minds – by replacing your old, worn out computers with intuitive space saving MCAN electronic data collection technology. Excite students in your general chemistry labs by using the same technically advanced instrumentation used by university research labs and real-world industry chemistry labs. Make your chemistry lab program exciting and technologically relevant to students by using electronic data collection technology in your labs.

The MCAN® Concept
The MeasureNet Multifunctional Chemical Analysis Network (MCAN® ) is an innovative analytical instrumentation eliminating a multitude of the obstacles that are associated with the PC-based lab systems. With the consolidation of student data acquisition workstations into a solitary network for each group of students, expensive hardware upgrades, computer viruses and the footprint of large equipment is eliminated.
The student workstations we provide interface with numerous kinds of probes as well as other apparatus that are utilized in chemistry laboratories. This allows for a broad range of general chemistry, physical chemistry and biochemistry lab experiments.
MeasureNet can be useful in a freshman chemistry lab or in advanced chemistry labs. Whether used in the freshman chemistry lab, biochemistry, STEM, or environmental chemistry laboratories, MeasureNet is an essential tool.
With MeasureNet, the student takes the measurements at their workstation and the results of those measurements can be stored and then monitored on a single central computer. This allows the instructor to follow student progress and data files. There is no need to contend with the multiple headaches that are associated with managing student lab data because MeasureNet can do it all for you.
Solutions in the Chemistry Lab with MeasureNet
- Less bench space due to energy efficient workstations with smaller foot prints.
- Securely Protected online data storage for students.
- Viruses and computer re-imaging are things of the past with MeasureNet.
- The collection of data electronically allows for more time being spent on the actual experiments.
- Eliminates the need to upgrade large numbers of computers every few years.
- The best quality, research grade chemistry probware is utilized to collect high-resolution data.
General Chemistry Lab Experiments Performed with MeasureNet
MeasureNet was designed to offer instructors considerable academic flexibility with its ability to support a wide range of experiments.
MeasureNet has the ability to support experiments for individual or collaborative student projects in a freshman chemistry lab or in an advanced lab.
The adoption of electronic data acquisition does not require the disposal of experiments that have been proven to build the students’ skills or enhance their understanding of imperative concepts. The longstanding experiments can very easily be integrated into curricula combined with the experiments appropriate for MeasureNet. Other experiments requiring conversion can be modified with the assistance of our curriculum specialists.
Title examples for Guided inquiry, Self-Directed, POGIL, Verification-style and STEM experiments utilizing MeasureNet are as follows:
- Specific Heat of a Metal
- Hot & Cold Packs- Dystan Medical Supply Company
- A Colligative Property of Solutions-Freezing Point
- Quality control at the GlassEX Company-Self-Directed
- Buffer & pH Solutions
- Proteins & Amino Acids
- Chemical Kinetics
- Gas Laws
- Voltaic Cells
- Substances Specific Heat
- Analysis of Metals Emissions
- Heat of Vaporization & Vapor Pressure
- Determining Chromium (VI) Concentrations using Absorption Spectroscopy
- Colligative Properties
- Determining the Cause of a Fish Kill Located in the Clark Fork of the Columbia River
- Identification of a Weak Unknown Acid
- Analysis of the Phosphorus in Cola
- Identification of an Unknown Metal-Self Directed
- Determining the Ka Value of a Weak Acid
- Determining the concentration of Acetic Acid in Vinegar
- Determining the Molecular Weight, with the use of the Ideal Gas Law, of a Volatile liquid
- Hess’ Law-Enthalpy of Reaction
- Analysis of the Phosphorus in Fertilizer
- Determining the Heat of Neutralization for Various Strong Bases & Acid
- Reaction Stoichiometry & Moles
- Determining a Reaction Equilibrium Constant with the Use of Absorption Spectroscopy
- Analysis of Emission of Aequeous Solutions from Group IA & IIA Metal Salts

Stanislao Cannizzaro (1826-1910) Professor at Alessandria (1851-1854), Genoa (1855-1859), Palermo (1860-1869) and Rome (1870-1910), Cannizzaro is best known for his discovery of the Cannizzaro reaction in organic chemistry for the conversion of aromatic aldehydes into the corresponding acids and alcohols, and his successful resolution of the atomic weight problem. Though Dalton had introduced the idea of extracting atomic weights from combining weights in 1803, he was unable to do this in a completely unambiguous fashion and, as a result, 50 years of chaos followed during which chemists used a variety of competing atomic and equivalent weight values. In 1858 Cannizzaro published a small pamphlet in which he reasserted Avogadro's earlier hypothesis (1811) that gas densities at equal pressures were directly proportional to molecular weights. Whereas Avogadro had attempted to extract atomic weights from the resulting molecular weights by using the stoichiometries of gas reactions - a procedure that could be applied only to a few elements - Cannizzaro showed how this same information could be extracted by using the gravimetric composition of an element's volatile compounds - a procedure that was virtually universal. With Cannizzaro's advance, chemists finally acquired a standard set of atomic weights and were able to determine unambiguous and universally accepted compositional formulas for their compounds.
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati
Measurement of molecular fluorescence is an important analytical technique in chemical and biological sciences. The capacity of detecting very small fluorophore concentrations, or small changes in its concentration, combined with high specificity make this technique a very powerful analytical tool.
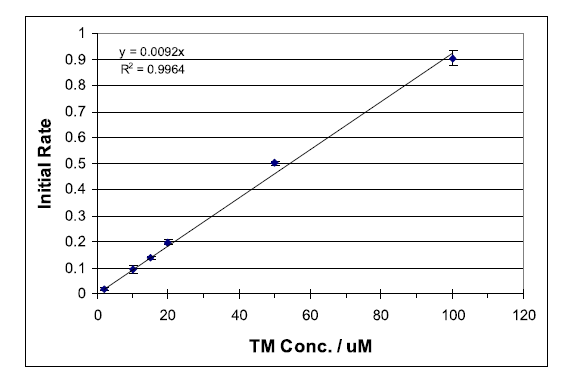
Kinetic methods for determining reaction rates are commonly used. While most experiments are designed to determine the order of a particular reaction in order to gain insight to the reaction mechanism, kinetic methods are also used for quantitative analysis. Determination of the initial reaction rate is one way of quantitatively analyzing a compound within a suitable reaction system.
The following experiment is designed to introduce students to both of these concepts, fluorescence and kinetics as analytical method. This combined technique has the advantage of increased analyte specificity over equilibrium-based fluorescence measurements. As only the compound that is reacting is causing a change in the measured fluorescence signal, steady-state interferences are largely eliminated.
Thiamine (vitamin B1) is essential for the metabolism of carbohydrates and normal function of he nervous and cardiovascular systems. Severe vitamin B1 deficiency will eventually lead to beriberi, characterized by abnormal functions of the muscular and nervous systems, as well as heart and brain abnormalities. Vitamin B1 occurs naturally in foods like whole grains, nuts, vegetables, pork, and liver.
Thiamine (TM), a non-fluorescent compound, has been found to be oxidized selectively by mercuric oxide (HgO) at a rate suitable for monitoring with standard fluorescence spectrometers.1 The oxidation product, thiochrome (TC), is fluorescent with a strong absorbance maximum at 367 nm and fluorescence emission at 444 nm. Deprotonation of TM yields a non-fluorescent tricyclic intermediate (CI), which is oxidized to TC as outlined in Scheme 1. Immediate oxidation of CI is essential to avoid the formation of several non-fluorescent productions.1 Thus, it is important to follow the sequential addition of reactants as outlined below... To view the complete experiment Click Here.
Svante Arrhenius (1859-1927). Swedish Chemist. Lecturer and Professor at the Technical Institute in Stockholm (1891-1904). Nobel Prize Winner (1903) and later Director of the Nobel Institute (1905-1927). Arrhenius is best known for his theory of ionic dissociation, which evolved out of his doctoral thesis of 1884, his ionic acid-based definitions (1887), and his introduction of the concept of activation energy in chemical kinetics (1889). He later did pioneering work on the physical chemistry of serums, ecology (where he is responsible for much of our early understanding of the greenhouse effect), and cosmology.
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati

"Gas"
Joseph Priestley (1733-1804). British chemist and Unitarian Minister. Priestley earned his living as a minister and teacher at various dissenting academies. His work in chemistry was done in his spare time and largely during the period when he served as private librarian to Lord Shelburne. It dealt almost exclusively with the use of the pneumatic trough to discover a large number of new "airs" or gases, including nitrogen oxide, nitrogen dioxide, dinitrogen oxide, ammonia, hydrogen chloride, sulfur dioxide and, most famously of all, oxygen (1774). He also investigated the processes of brewing, photosynthesis, respiration, and invented soda water. Priestley was a prolific writer, not only on chemistry, but also on the subjects of theology, history, geography, natural philosophy, and electricity. Due to
his liberal political opinions, his home and laboratory were destroyed by a Birmingham mob in 1791, and he spent his final years in Northumberland Pennsylvania, where he wrote several pamphlets defending the outdated phlogiston theory against Lavoisier's newer oxygen theory of combustion.
Courtesy of Professor William Jensen, Oesper Chair of the History of Chemistry and Chemical Education, University of Cincinnati
 LUMINESCENCE/PHOSPHORIMETRY
LUMINESCENCE/PHOSPHORIMETRY
Last, but certainly not least, is the use of the MeasureNet “Colorimeter” as a “Phosphorimeter”… also called a “Luminometer”… which, is one of the more versatile Analytical “tools” not readily found in most laboratories. A “Phosphorecent” response can be induced by UV-fluorescence AFTER the light souce is removed… or it can be caused by a chemical reaction that causes the “emission” of VISIBLE Light photons. The proprietary design of the “Colorimeter” optical layout allows the analyst to get the desired response from their sample material… again using the same high-sensitivity optics.
Bio-Luminescence in Fish and Algae, Luciferin/Luciferase Reaction in Fireflies, UV-Blocking capacity of SPF Lotions, the ‘controllable' oxidation of Luminol Light-Sticks, the newly developed photo-luminescent additives for plastics used in skyscraper building and more are beginning labs you can use directly as-is, or modify for your own specific curriculum… as outlined for your review:
Luminescence (“Phosphorescence”) for Kinetic & Relative Comparison values:
• Check out these Glow-in-the-Dark Plastics… some are made of material quite Fantastic!
• Bio-Luminescent Algae and Bacteria are found in “Healthy” Waters… See anything “Fishy”?
• P-AminoBenzoic Acid is the SPF of “Old”… using Luminscence its tale can be told!
• “UV” Light causes “Glow-in-the-Dark” Photon Motions… “Block” it with some SPF Lotion!
• “Luciferase” is the enzyme in the Fire-Fly… catch a few & give this Phosphorimetry Lab a try!
• The Luminol “Light Stick” reaction… uses Organic Oxidation to set those Photons in action!
Many current MeasureNet users that have the MDBC-138 “Colorimeter”… and potential customers, too… may not be aware of the tremendous versatility of this spectral device… and many potential *new* Customers in academia can save a significant amount of time, money and resources by implementing this technology for their teaching laboratories and educational curricula. Designed for simple, direct plug-n-play capability with any MeasureNet Workstation.
The Princeton Section of the American Chemical Society will be holding their annual National Chemistry Week Activities Night On Friday evening, October 21 from 7-9 pm. It will be held at Princeton University, Frick Laboratory, located on Washington Road, Princeton NJ. This year's theme is "Chemistry - Our Health, Our Future!
A week of fun and educational activities are planned in
Manhattan to showcase how chemistry makes life better. The events are part of National Chemistry Week, Oct. 16-22, and are sponsored by Kansas State University's local section of the American Chemical Society and the department of chemistry.
We will celebrate IYC 2011 at our yearly NCW Event held at the NY Hall of Science on Sat., 10/22! Local universities and companies will have a variety of cool hands-on demos for kids ages 5-15! We will also host a "Chemistry Bingo" where winners will win an IYC 2011 lapel pin and other neat goodies!
Enjoy classic chemistry experiments and learn the positive impacts of chemistry as it relates to nutrition, hygiene, and medicine.
10:00 - Chemistry activities, Protozone - Level 1
11:30 - Chemistry activities, Protozone - Level 1
1:00 - Cool Chemistry Program, Demonstration Station - Level 1
The No. Jersey Section's NCW celebration and student poster session will take place at a
ChemExpo celebration at Liberty Science Center on
Saturday, October 22 from 10:00 AM to 2:00 PM.
Saturday, Oct 22 10:00a to 1:00p
The Santa Clara Valley section of the American Chemical Society will hold a National Chemistry Week Celebration for kids of all ages. The Wheel of Chemistry Fortune will be spinning for all kids to win a prize, and there will be fun hands-on chemistry including a slime lab! This will also be an opportunity to pick up a free copy of “Celebrating Chemistry”, the NCW newsletter for elementary-aged children.
The New York Section of the American Chemical Society will be celebrating National Chemistry Week on October 22, 2011 at the Great Hall in the New York Hall of Science. This day-long event will showcase chemistry principles using demonstrations performed by local college students and volunteers from local industries for children of all ages. This year's theme is "Behind the Scenes with Chemistry" and the event will run from 11 am to 4 pm.
As part of the National Chemistry Week 2011 celebration, the International Year of Chemistry (IYC) and in recognition of its theme, "Chemistry - Our Health, Our Future!" The American Chemical Society (ACS) and the Department of Chemistry at Texas A&M University are sponsoring an illustrated poem contest for students living in the Brazos, Robertson, Burleson, Washington, Grimes, Madison, and Lean Counties in grades K-12.
National Chemistry Week, sponsored by the American Chemical Society, is celebrated at Gordon College by our department. See some of the different ways students and faculty get involved.